Abstract
BACKGROUND: B-type natriuretic peptide (BNP) is a hormone secreted in response to volume-related ventricular stretch, and as a biomarker is both diagnostic and prognostic in heart failure. Elevation in BNP or its equimolar N-terminal prohormone fragment (NT-proBNP, noteworthy for its longer half-life) qualifies as a criterion in ISBT-IHN-AABB guidelines for the diagnosis of transfusion-associated circulatory overload (TACO). However, real-life range comparisons in TACO vs. non-respiratory transfusion reactions (TR) are lacking. In the Transfusion Associated Dyspnea-Prospective Observation and Laboratory Assessment (TADPOL) study of acute TRs requiring laboratory investigation (cardiorespiratory events +/- fever [CRTR+/-F] vs. standalone high-risk fevers [HRFTR]), patients consented to NT-proBNP testing as part of a multi-dimensional assessment of presentation archetypes and final diagnoses (e.g., TACO, transfusion-associated dyspnea [TAD], febrile non-hemolytic TR [FNHTR]).
METHODS: Blood samples were collected within 24h of reaction onset from patients enrolled across 6 centers in the study's first 134 weeks. NT-proBNP (ng/L) was quantified by chemiluminescence immunoassay (Roche Diagnostics) in real-time (3 sites) or by batched testing (3 sites); reference cut-off values to rule in a cardiac cause for dyspnea in the acute care setting were >450 (age <50y), >900 (age 50-75y), and >1800 (age >75y). Summary statistics are reported. Relationships to provisional reaction diagnosis and clinical features were also explored using appropriate tests (Student's t-test, Mann-Whitney U, Pearson's correlation, and/or Fisher's exact test). Discrimination between possible-to-definite TACO and doubtful or ruled-out TACO was assessed using receiver operating characteristic (ROC) curve analysis with logistic regression.
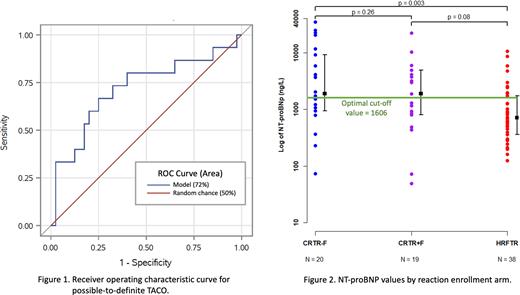
RESULTS: At the study mid-point, 1180 TR (604 TADPOL-eligible) occurred, with NT-proBNP tested in 77/100 enrolled patients. NT-proBNP was significantly higher in patients enrolled in the CRTR arms compared to HRFTR (p=0.0009), with no difference between CRTR+F and CRTR-F (p=0.4). NT-proBNP was also significantly higher in patients with dyspnea in the TR (p=0.005). There was no correlation between NT-proBNP and systolic blood pressure change (p=0.1) or at baseline (p=0.1). Provisional reaction diagnoses were available for 55 patients including 32/39 in the CRTR arms: for possible-to-definite TACO (n=14) and TAD (n=19), vs. standalone FNHTR (n=38), the median NT-proBNP (IQR) was 3,439 (1,587-10,668) and 1,893 (674-5,361), vs. 710 (124-10,542), respectively. Transfusion-related acute lung injury was considered possible in 3 cases, all with TACO. Among CRTR, there was a trend towards higher NT-proBNP values in patients with TACO (p=0.05), with no difference in ages (p=0.8). Using the age-adjusted reference cut-off, the sensitivity of high NT-proBNP for TACO was 86%; specificity was 54% when differentiating TACO from all enrolled TR and 38% between TACO and non-TACO CRTR. In ROC curve analysis (Fig. 1), the area under the curve was 72% (95% CI 55-88%) and the threshold value for discriminating TACO from other TR was 1606 with 60% sensitivity and 65% specificity (Fig. 2).
CONCLUSIONS: NT-proBNP is strongly correlated with dyspneic TRs compared to isolated febrile TRs. Elevations appeared more marked in TACO, with a sensitivity of 86% using existing laboratory reference values. However, specificity and discrimination within CRTR were poor. These results affirm that NT-proBNP is helpful in ruling out TACO, whereas ruling in TACO continues to demand a more integrated approach. Whether cardiac strain in unclassified CRTR and TAD is evidence of missed TACO or other insults remains to be determined.
Disclosures
Pavenski:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in clinical trials, industry-sponsored educational events; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participated in clinical trials, industry-sponsored educational events; Roche: Other: Participated in clinical trials.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal